CURRICULUM
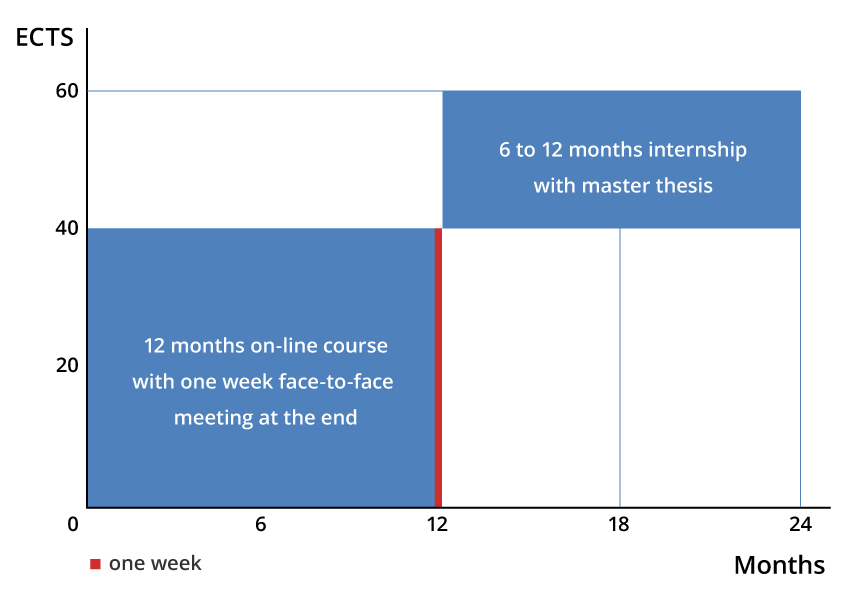
- The duration of IMVACC is 1.5 to 2 years with the first year dedicated to teaching and the second year to an internship and a master thesis.
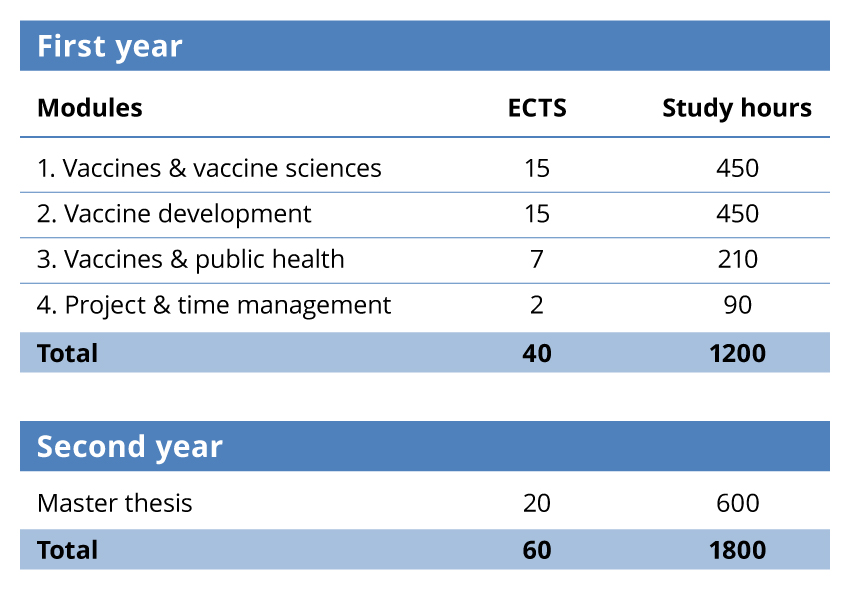
- The 60 ECTS are distributed as follows:
IMVACC consists of 2 parts, a theoretical part during the first year with 4 modules with the European Credit Transfer System ( ECTS) credits and the study hours allocated to each module and a practical part in the form of a master thesis project under the supervision of a local thesis director.
- The curriculum is valued at 60 ECTS (in conformance with the European Credit Transfer System) and the credits are distributed as follows
During the 12 months online course the students study the 4 modules and answer the questions associated to scientific or clinical articles and case studies. A 2 hour session using virtual meetings with the tutors is organized biweekly. Each module ends up with an exam. At the end of the first year there is a 3 day face to face meeting during which the students complete the final exam. At the end of their master thesis project, the students write a report and defend their thesis work during a online session in presence of the jury composed of the IMVACC tutors and the thesis director.
MODULE ORGANIZATION
IMVACC is constituted of:
- Four modules of online content, activities and assessments
- A master thesis
It is preceded with an assessment of the knowledge of students in immunology, bacteriology and virology (prerequisites)
Vaccines & Vaccine sciences
This module reviews human immunology, the immunology of vaccines, and specific general vaccinology topics including the types of vaccines, the routes of administration and delivery devices. It also presents the history of vaccines as well as information on most of licensed vaccines and vaccines currently under development. Each vaccine is presented in the context of the disease to be prevented, its epidemiology and of the causative pathogen. If you want to know more, the module offers also concepts of statistics and epidemiology.
Activities include annotated articles on various topics of vaccine immunology, the study of the first text of Edward Jenner on his experiments with cowpox to protect against smallpox, and team work on the development of vaccines against Staphylococcus aureus.
Vaccine development
This module first presents an overview of the whole vaccine development process, from discovery to launch. It is followed by a discussion of the different fields of vaccine development activities, including quality management:
- research leading to the identification of protective antigens and of how a protective response can be elicited (discovery),
- assessment of vaccine candidates for safety and efficacy in animal models (preclinical testing),
- development of a vaccine manufacturing process, first at pilot scale (process development) and then at industrial scale (manufacturing),
- testing of vaccines in humans (clinical development), including how immune responses are measured (immune response analysis),
- preparation of the registration file and the reviewed process by regulatory authorities prior to licensure (regulatory affairs).
In addition, as the vaccine development work is more and more shared between different entities, private and academic, the module includes a chapter on activity outsourcing.
Learning activities include annotated documents, exercises on how vaccines can be improved, on their clinical development, including how a protocol is written, on the content of a registration file as well as how the environmental impact of a genetically modified organism used as vaccine is assessed.
Vaccine & public health
The module presents various topics related to public health. Among them are:
- resistance to vaccination and its evolution over time as well as the vaccination of health care personnel,
- impact of vaccine use on disease burden and how it is measured,
- elements of pharmacoenconomics,
- how vaccines are made available to people who need them,
- information on vaccine markets,
- how the safety of vaccines is assessed and followed during the post-marketing stage, and
- emerging diseases.
Activities include document reading as well as individual and team work on the process of rapid risk assessement of acute infectious diseases as defined by WHO, as well as the development of an action plan to increase the vaccination coverage against influenza among employees of a supermarket.
Project & time management
Vaccine development activities are complex and multidisciplinary. They have a defined and precise goal and are limited in time. They are best managed as projects using a methodology that was defined to maximize the chances of meeting the goal of the project within time and budget while minimizing the risk of failure. This module will present the main project management processes as well as ways to avoid pitfalls.
The basic concepts of project management, including time management tools, will be studied at the beginning of the course. They will be useful to plan study time and to develop the master thesis project.
At the end of the first year, students will have a global view of all facets of vaccinology, from scientific to engineering aspects. The goal of the second year is to use that knowledge to define and execute a personal project. The deliverable is a master thesis that will be presented to a jury for graduation.
Tutors will assist the trainees in the definition of the master thesis subject. They will also be available for discussion and guidance during the execution of the project.
Students without professional experience may acquire practical experience in a specific field of vaccinology by working in a vaccine company, a research laboratory, or a regulatory agency.
Students who already work in the field of vaccines will either broaden their experience and professional knowledge, or acquire competencies in a new field.